Acid Base Equilibrium Practice Test
Practice calculations involving solutions of weak acids and bases in this set of free practice questions designed for AP Chemistry students. Whose definition of acids and bases emphasizes the role of protons.
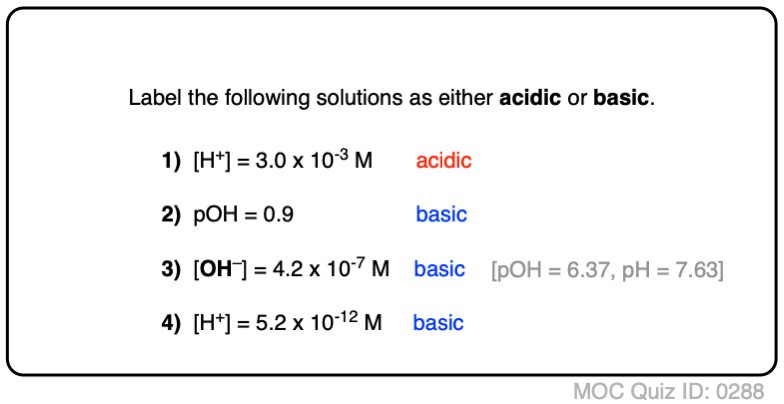
Acid Base Practice Problems Master Organic Chemistry
Chemical processes Acidbase equilibria.
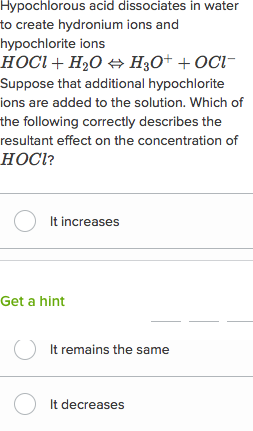
. NH 2 CONH 2 aq 3. Choose your answers to the questions and click Next to see the next set of questions. Download my free guide 10 Secrets to A.
Acid-Base Equilibrium Quiz This online quiz is intended to give you extra practice in calculations involving weak acids and bases including K a K b pH and ion and solution concentrations. A 0500 mole sample of which compound below when placed in water gives the lowest concentration of NO2-1 ions. NH 4 and NH 2.
Which of the following is a conjugate acidbase pair. Heres a quiz shows that its actually easier than it looks. All acids and bases are good electrolytes.
Select your preferences below and click Start to give it a try. AcidBase Equilibria Exercises is shared under a not declared license and was authored remixed andor curated by LibreTexts. PO43 aqH 4.
The ABC test is actually easier than many people think because the only elements that occur naturally are gases and liquids. Which is the weakest acid. Brønsted and Lowry c.
SCH 4UI UNIT 4. The acid has one more proton than. Test prep MCAT Foundation 5.
Which acid or base is not a good electrolyte. Two species related to each other through the gain or loss of a proton. Answer choices HF HNO 2 CH 3 COOH HClO Question 2 900 seconds Q.
NH 3 and NH 4 d. H 2O and O 2 b. Kab -14for a conjugate acidbase pair Quadratic Equation.
Instead were using CARIO to determine which acids and bases are stronger and predicting the reaction direction accordingly. Reactants and products are constantly going back and forth b Its a steady state. Ka and acid strength.
Answer choices HClOCl - H 2 SO 4 SO 42- NH 4 NH 3 H 3 O OH - Question 3 900 seconds Q. Practice Study Guide Course Acids Bases Equilibrium Chapter Test Prep Plan - Take a practice test Acids Bases Equilibrium Chapter Exam. NaF and F c.
Strong bases are a. The concentrations stay steady once equilibrium is established. What is the conjugate acid of H2PO4aq.
There are four types of elements and they come in the form of AB a and b. 729x10 The concentration of sodium hypochlorite in household bleach can be determined by titration with a reducing agent such as iodide solution. THE NATURE OF ACID-BASE EQUILIBRIA P528 549 TOPICS Brønsted Lowry Theory Reversible Acid-Base Reactions The Autoionization of Water Strong Acids and Strong Bases Hydrogen Ion Concentration and pH pOH and p K w.
H 3O and OHe. NH 2 -and NH 4 8. Question 1 900 seconds Q.
OCl aq 2I - aq 2H 3 O. Which one of the following is a conjugate acidbase pair. Which of the following is false about a system at equilibrium.
Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C. Which one of the following is a conjugate acidbase pair. Calculate the base ionization constant for hypochlorite The K a value for hypochloric acid is 35 x 10 -8 K b ClO- equals.
This video walks you through a few practice questions where youre asked to determine the position of equilibrium for acid-base reactions WITHOUT using PKA values. Science Courses OSAT Chemistry CEOE 004. An electron-pair acceptor is a a.
Up to 24 cash back 1. Organic Chemistry Acid Base Equilibrium Practice QuestionsNeed help with Orgo. Chemistry of buffers and buffers in our blood.
Chemical Equilibrium Acid-Bases Chapter Exam Exam Instructions. X a bbac 2 24 1. BCl3 CH32O BCl3 OCH32.
What is the pH of a 035 M aqueous solution of HN3. This reaction is similar to that in which a proton is added to ammonia as it also involves a Lewis acid and a Lewis base interaction. Acid Conjugate Base H By this definition HNO2 NO 2- H CH 3 NH 3 CH 3 NH 2 H C 6 H 5 COOH C 6 H 5 COO - H H 3 O H 2 O H We can see that the conjugate base of H 3 O is H 2 O.
CHEMICAL SYSTEMS AND EQUILIBRIUM CHAPTER 8 ACID-BASE EQUILIBRIUM LESSON 81. A Its a dynamic equilibrium. You can skip questions if.
Calculate the pH of a 047M NH 3 K b 18 x 10 -5 solution. A model of acid-base behavior based on proton transfer in which an acid and a base are defined respectively as a species that donates a proton and one that accepts a proton. The goal is to figure out what a noun is which comes in two parts.
But it is given that OH - is.

Acid Base Questions Practice Khan Academy

Acid Base Equilibrium Practice Organic Chemistry Youtube

Acid Base Questions Practice Khan Academy
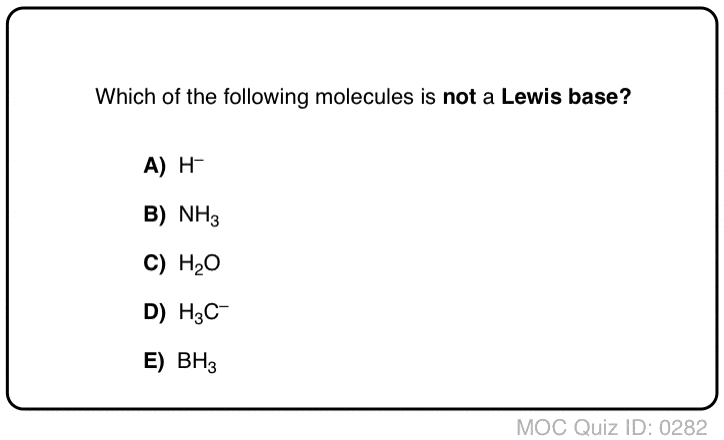
Acid Base Practice Problems Master Organic Chemistry

Acid Base Equilibrium Practice Organic Chemistry Youtube
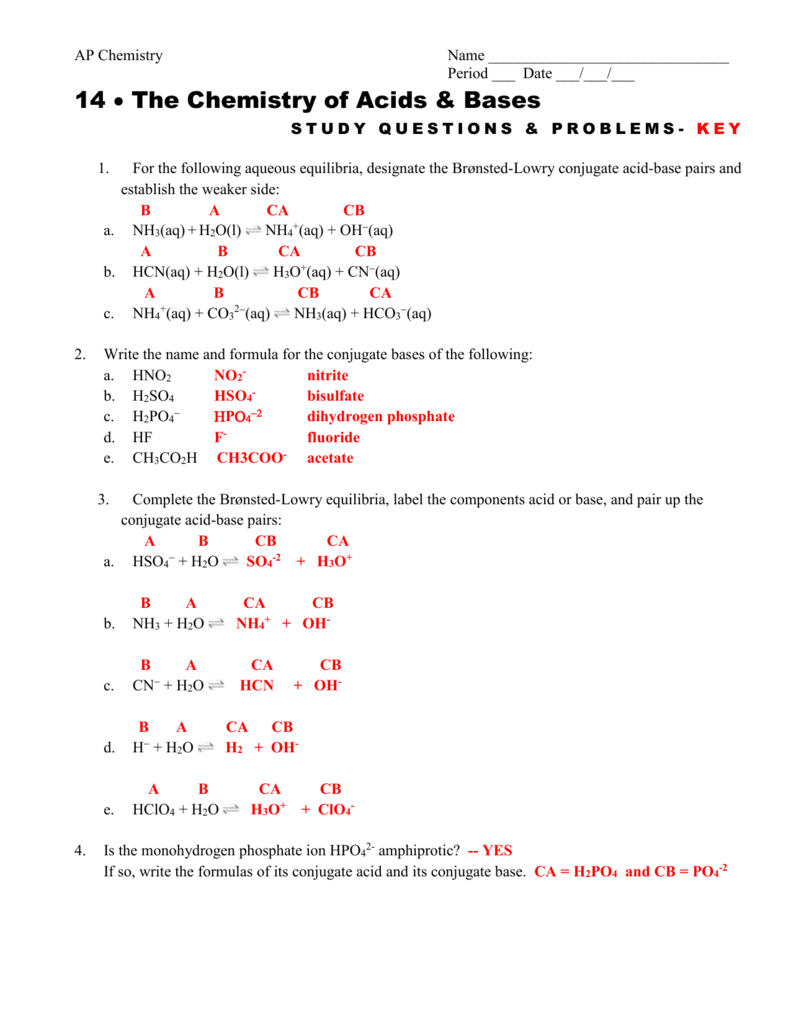
Chapter 17 Study Questions And Problems
0 Response to "Acid Base Equilibrium Practice Test"
Post a Comment